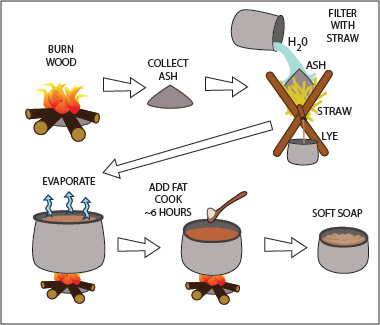
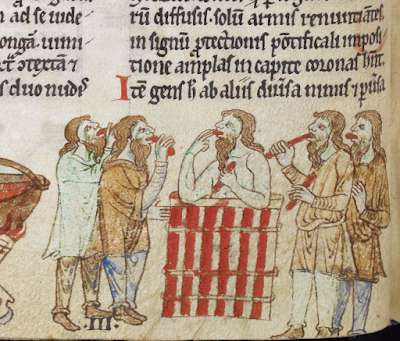
Irish Garb Part 1

Early Medieval Irish Garb Part 1: Analyzing Sources Background During the prehistoric period in Ireland, the insular Irish developed unusual laws, clothing and habits, relatively independently from the Continental trends of the time. Thanks to their island status, their material culture seems to have continued with few changes for many centuries, from the Iron Age onward. According to Barry Raftery, the Irish had a tendency to thoroughly assimilate anything new into their existing culture, art, and economy, without themselves being much changed by its adoption: “this illustrates well the recurring conundrum of Irish prehistory. Here we have something that is totally new in the country, yet rendered in a form that is different in detail from anything in the area of presumed origin.” (1) Thus, the Irish managed to hold on to a pastoral economy of grain, pig, and most importantly, cattle-raising, with a dispersed population on decentralized family holdings and a tribal style o...