Lye Part 2
Lye Part 2: Potash
To read the first post about the history of lye, see the previous post: Lye Part 1.
Potash lye
Potash, in this context, refers specifically to the ashes made by burning woody plants. Ideally, the firewood should come from hardwood species of tree: beech, mulberry, oak, locust, ash, elm, maple, birch, etc. Fresh potash is basic, typically around 11.6 pH (1). The ash is then soaked in water and filtered out, leaving a weak lye; this process is called leaching. Repeated leachings through fresh ashes can add to the strength of the lye, or the weak lye can be simmered to quickly evaporate away water and concentrate the lye.
Potash also refers to the chemical compound, potassium carbonate, which traditionally came from hardwood ashes. In older literature, potash could also refer to dehydrated potassium-hydroxide (the ashes left in the pot after hardwood ash leachate is boiled off). Since all three substances will be discussed here, potassium carbonate and potassium hydroxide will be called by their scientific names, and the ashes of hardwood will be called potash.
The many historical uses for potash
Bucking hides: Potash can be spread directly onto a fresh hide after it is fleshed, and used to prevent decay while causing the cells of the skin to swell. The alkalinity causes the follicles to release the hairs, loosening the fur of a hide so that it can be easily scraped clean. Once the hide has been dehaired, and sometimes grained (the outer layer of epidermis scraped away as well), it can be softened and oiled for immediate use (oil tan); softened, oiled and smoked (buckskin); stretched and sanded (parchment); or soaked in tannins for months to make leather. Potash is one of the safer and most readily available materials for bucking a hide, and may have been the first chemical treatment of skins known to man.
Food preservation: Layering meat in a bed of dry potash preserves the meat from bacteria, bugs and other pests that would ruin it; this practice continued in the Appalachian mountains as recently as a few decades ago. The ashes would be poured into the bottom of a corn crib or a wooden slatted box to form a thick layer; then the joints of meat would be placed into the ashes without touching each other or the sides of the box, and more ashes poured on top of them until they were completely covered; additional layers could be added so long as the meat was always fully enclosed by ashes. The meat had to be rinsed when it was removed from the layers of ash, and would taste salty, but would be safe to eat (especially if it was only kept during the winter months). Lutfisk, in the Scandinavian cultures, takes advantage of this attribute of ashes—although the fish is also allowed to ferment.
Lye has also been used as a traditional pre-cooking and preservation method for maize corn in the Americas. The dry kernels of corn are separated from the cob, and soaked in a strong potash lye solution. This forms hominy; a modern variant makes use of pickling lime for the same purpose.
Metalworking: Potash works as a purification agent in smelting and refining metals such as gold, silver, and tin. In its role as a flux, it helps purge impurities (slag) from the metal, and prevent the formation of metal oxides. Potash flux also saw use in brazing and soldering, especially in silver work.
Glass: As an alkali flux, potash lowers the melting point of silica—making it feasible to work glass at temperatures achievable with charcoal kilns. Medieval Europeans began to use potash from beech trees as a flux around the seventh century (2), as the import of natron from Egypt became prohibitively expensive with the decline of Roman trade routes, and increasingly dangerous, with the rise of the Islamic empires. By 800 CE, potash was the primary glass-working flux of northern Europe (3). Forest glass, so called because potash was produced in local forests, remained popular for many centuries because it actually brought the melting point lower than soda-ash, quicklime or natron; soda flux makes glass workable below 1,000ºC, but potash lowers the melting point to 750ºC (3). This meant that glassworks were easier to build, since they needed less advanced kilns and fuels.
Potash made glass heavier than other alkali, and frequently resulted in tinted, cloudy glass (especially if iron-rich sand were used, for silica, which would make a rich green color). Since forest glass was harder than other glass, it was better for cutting and etching, and held up better as window panes (3). The glazing of windows that spread so rapidly in the Medieval Period resulted directly from the cheap availability of this sturdier glass.
Agricultural: Potash works like agricultural lime, raising the pH of soil from acidic to basic (sometimes called, 'sweetening') to accommodate the preferences of many crops. It adds minerals and metals that may be lacking in a garden, especially if soil has been stripped by growing crops too many years in a row without soil amendments to replace the nutrients they extract (especially calcium). Potash is also sometimes added to livestock feed, or offered as a dry free-feed supplement, since it contains minerals extracted from mature trees—minerals that shallow-rooted plants may not be able to reach in a pasture.
Detergent: Potash lye on its own has been in use as a cleansing shampoo for human hair for millennia; however, its detergent ability reaches far beyond simple hygiene. Lye can be used to clean wool fleeces, preparatory to combing and spinning the wool—or to clean the finished, woven textile and begin the fulling process. Lye can be used to make felt, instead of soap. Potash lye works to clean dishes at a campfire, or to scrub a floor, or myriad other tasks; it simply requires a thorough rinsing, after the scrubbing, with clear water
Soap: Potash lye does NOT make hard bar soap. This is important for understanding the nature of soap in history. Most soap made in rural communities or by poorer urban residents was either a thick liquid, or a soft paste. This soft soap needed a container, like a clay jug, a trough or a barrel, and would be scooped out for use.
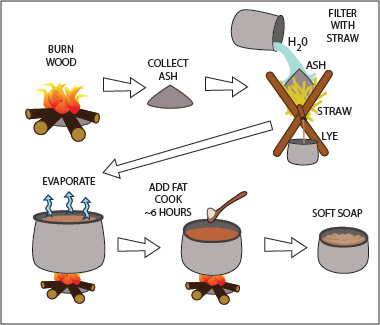 |
| The simplest soap: burn wood, soak & filter the ashes with water, evaporate off the excess liquid, add tallow and cook. |
Since the mineral and alkali content in each batch of potash lye varies, the soap made from it is not standardized. Depending on the plant burned to make the ashes, it may contain more sodium hydroxide than usual, which forms particles of hard soap in among the batch of liquid potassium soap, resulting in a thicker paste or cream soap. Alternatively, it may contain less total alkalinity than usual, and thus end up with more fat left over than normal—or it may be more basic than usual, exhausting the available fats and leaving some lye unreacted, making a harsher soap. Also, potash lye contains other minerals and metals, depending on the soil where the tree grew, lending different qualities to the resulting soap.
Thanks to these variations and the expertise required to get a consistent product, soap-makers from particular regions gained fame for the quality of their soaps (see the history post, Lye Part 1). Part of the success of a region's soap industry depended upon the availability of better ash: beech trees, for example, were abundant in what is now Germany, and beech tree ash resulted in a much stronger concentration of potash in the first leaching (3). Elsewhere, the availability of certain other plants (especially barilla and seaweed) made it possible to produce soda ash, containing much more sodium hydroxide and resulting in hard soaps. Where useful plants for potash were less abundant, historical cultures usually relied upon other alkaline cleansers and detergents (natron, lime, fermented urine, etc).
Chemistry of potash lye
Burning wood produces about 5% ashes, relative to the original mass of dry wood—averaging 5% ash (4). The combustion of wood is an oxidation reaction, creating CO and CO2, and leaving behind metals and minerals, primarily: calcium, potassium, phosphorous and magnesium (5). The concentration of oxides and residuals in the ash varies according to the species and growing conditions of the trees, the temperature and method of burning, and the storage of the ashes. Typically, calcium carbonate (CaCO3) makes up a large portion (25-43%) of the resulting ashes, while only a small amount (often less than 10%) is “true potash.”
“True potash” is potassium carbonate (K2CO3), an alkaline salt that is sometimes called pearl ash or tartar. The species of tree burnt to make ashes will affect the amount of potassium carbonate dramatically. For example, beech tree ash contains, on average, about 20% potassium carbonate (4).
Different burning methods can also affect the yield of potassium carbonate. Higher temperature fires will produce less total ash, but incomplete combustion may leave unreacted carbon (charcoal) instead of ash. Sheltered combustion (as in a kiln) traps CO2 during the burn, holding more of it in place to react with the potassium in the ashes, to form additional potassium carbonate.
Water plays a vital role in extracting potassium carbonate, which is very water soluble, from the calcium carbonate, which is not. Water also converts some of the potassium carbonate into potassium hydroxide. This is crucial for the purpose of making lye:
H2O self-ionizes naturally, producing a quantity of H+ and OH- ions in equal portions.
Potassium carbonate is a soluble ionic compound; it dissociates when fully dissolved in water. During leaching, some of the dissolved potassium carbonate splits into potassium cations (2K+) and carbonate anions (CO3 2-).
Some of the carbonate anions (CO3 2-) abstract with the H+ ions in the water to make bicarbonate anions (HCO3-).
Some of the potassium (K+) and hydroxide (OH-) ions bond to form potassium hydroxide (KOH). (4)
This solution of potassium carbonate (K2CO3), potassium hydroxide (KOH) and other dissolved minerals and alkali (depending on the source ash) is called potash lye, or just lye.
It is hard to guess in advance exactly what proportion of each chemical will be present in a batch of potash lye. It may contain more potassium carbonate, or more potassium hydroxide, depending on the type of wood and the circumstances around its growth, burning and storage. This means that sources vary EXTREMELY widely on the subject, claiming everything from “no potassium hydroxide was present” to “very little carbonate remained.” For example, Campbell's 1991 paper stated, “wood ash leachate contained 92% hydroxide and 8% carbonate” (6), while Babayemi found in 2011 that sampled potash contained only “potassium carbonate, sodium carbonate and some chlorides and sulphates” (7).
It IS important to understand that potash lye derives some of its alkalinity from the potassium carbonate, not just from the potassium hydroxide. Soap-making literature often over-simplifies potash lye, as if it were exclusively potassium hydroxide. Modern hobby-soap-makers rely upon commercial KOH, dissolved in water, for their potassium lye soaps, but this will invariably produce a different chemical reaction and product, compared to using lye leached from hardwood ashes.
The conclusion to take away from this is that it is important to use good hardwoods (especially beech or mulberry) that have grown in fertile soil with plenty of potassium, and were burned in a sheltered fire pit or woodstove, and were leached immediately with pure water. Even then, the local variables may give different results—so the hobbyist attempting to leach potash lye should keep good records and learn to work with the material. Flexibility and understanding of the materials are key.
Potassium carbonate
As the main alkali in wood ashes, potassium carbonate is a base; it normally measures about 11 pH. It exists naturally, and can be extracted from hardwood ashes. Modernly, it is usually produced synthetically from potassium hydroxide (the reverse of the process being examined here).
Aside from its association with potassium hydroxide-soaps, potassium carbonate itself has uses as an alkaline stabilizer. In the nineteenth century, the leather-conditioner industry began to use it to make gentle saddle soaps—a function that it still performs today. According to a textbook on waxes, polishes and conditioners, “potassium carbonate is a saponifying agent far superior to the cheaper sodium carbonate. For example, when the manufactured creams are stored at low temperatures, sodium carbonate is apt to crystallize in fine needles, but potassium carbonate does not have this disadvantage. Soaps act not only as emulsifiers but also as stabilizers (protective colloids)... The saponification number of the wax (expressed in grams) is multiplied by 1.5, the result being the number of grams of technically pure 96-98% potassium carbonate required for each kilogram of that particular wax” (8). Although saponification of fatty acids by potassium carbonate is less complete than by potassium hydroxide, it clearly plays a role in soap-making, and as a saponifier on its own, it has its advantages.
This matters for understanding why potash lye from real wood ashes differs from the commercial KOH concentrate used by hobby-soap-makers today. Although the bulk of the saponification will use the potassium hydroxide in the solution, some will actually use the potassium carbonate and produce a soft, emulsified fatty soap in among the strong, clear liquid soap produced by the hydroxide. This is not the same as a super-fatted liquid soap (which will go rancid); the fats are truly saponified, stabilized so that they will not go rancid.
Potassium carbonate + lime
The goal of leaching hardwood ashes with pure water is to generate an aqueous solution of potassium hydroxide, a much stronger base than potassium carbonate. Though potassium carbonate is capable of saponification, potassium hydroxide saponifies fats faster and more thoroughly.
One way that the earliest recorded makers of soap (see Lye Part 1) ensured that their wood ash leachate would contain sufficient potassium hydroxide was by adding caustic lime. This was a common alkali produced across the ancient world, with many uses. It mas made of limestone, baked at high temperature in a kiln, and crushed/powdered, then slaked in water. Quicklime, hydrated lime, slaked lime and caustic lime are all rather ambiguous names for this and other closely related products. Naming conventions differ, and standardization has yet to fully clarify which term refers to which product. One simple way to think of it is that lime (barn lime, agricultural lime, crushed limestone, calcium) becomes caustic lime (quick lime, calcium oxide) when it is heated, and then hydrated lime (slaked lime, calcium hydroxide) when it is soaked.
 |
| Lye + lime: an additional step that ensures stronger, more liquid soap. |
Adding calcium oxide to the lye converted more of the potassium carbonate into potassium hydroxide (9). The calcium oxide (CaO) bonded with water to become hydrated lime (Ca(OH)2). Hydrated lime reacts with potassium carbonate (K2CO3), producing calcium carbonate (CaCO3, which drops out of solution—as mentioned above, calcium carbonate is not very water soluble) and potassium hydroxide (KOH).
CaO + H2O → Ca(OH)2
Ca(OH)2 + K2CO3 → CaCO3 + KOH
So, early soap manufacturers in Gaul included the use of caustic lime to guarantee that there would be more potassium hydroxide in their lye than potassium carbonate, regardless of the variations in their wood ashes. Later soap-making evidence is mixed; not all recipes call for lime, but some do. Perfectly functional soft soap can be made with plain potash lye. However, a clearer and thinner liquid soap results from increasing the potassium hydroxide content of the lye, and caustic lime is an effective way to accomplish that.
Potassium hydroxide
Potassium hydroxide, firstly, is NOT interchangeable with sodium hydroxide! KOH ≠ NaOH. Potassium hydroxide cannot be substituted into recipes written for sodium hydroxide to make soap, even though both are called lye. Different alkali saponify fats differently! Potassium hydroxide reacts differently with various fats, and it needs correspondingly different measurements to achieve full saponification.
Furthermore, potash lye is not pure; it contains other alkali and minerals that may change the resulting soap. A hobby-soap-maker who has lots of experience using sodium hydroxide will need to adjust away from the strict, measurement-heavy recipes to be able to work with potash lye, because it requires a mindset of observant flexibility. Trial and error may be necessary to get the right balance of fats for the specific ashes available to the individual soap-maker.
Next, potassium hydroxide is quite temperature stable. Potash lye can be simmered to evaporate water for the sake of increasing the alkali concentration. However, potassium hydroxide is not stable when left exposed to air. It bonds to molecules in the atmosphere over time, decreasing the amount of potassium hydroxide available in the ashes, or in the lye solution, or even in a store-bought container of dry concentrate. Care should be taken to store it in a sealed container, and to only open it briefly when needed. Ash will make the best potash lye if leached immediately, rather than stored, but if it cannot be used right away then closing the ashes into a coal hod with a tight lid (or a sealed plastic bucket) will help preserve its strength.
Potash lye will gradually etch glass. Potassium hydroxide does not do this as quickly at room temperature as sodium hydroxide, but given a long period of time, or if at boiling temperatures, it will react with the silicates and damage the glass. For long-term storage, use clay or PP plastic. For cooking soap, use stainless steel pots or ceramic crocks, not Pyrex or enamel. Never put lye in pots made of aluminum, copper, tin or other reactive materials.
Finally, a note on concentration. As a commercial powder, potassium hydroxide is typically diluted with distilled water at a 34% solution (1.33 g/mL density) for hobby soap-making. By contrast, the first leachings from beech wood ashes only contain 13-16% potassium hydroxide, and the first leachings from oak ashes have only 9.5% potassium hydroxide (10). At this dilution, the lye is much safer and gentler to work with (though it should still be rinsed off the skin quickly), but it does a very poor job at making soap.
Traditional ways to concentrate the potash lye include boiling, and repeated leachings of the lye through the same or additional ash. The latter method, however, seem to actually serve a clarifying role rather than much of a strengthening role (11). Susan Verberg (Mistress Elska) used this method and compared the pH of the lye before and after repeated leaching, with little change, but she did note that the dark liquid became lighter as more of the carbon particles were removed by repeated filtering.
To concentrate the lye, therefore, it should be evaporated by simmering the solution, uncovered, until it makes popping sounds (the bubbling eruptions in the thickened liquid at the bottom of the pot). There is also the option of continuing to heat and evaporate the solution until only the dry crystals are left in the pot (these salt flakes are the original “pot ashes”), and then re-diluting them with pure water when they are needed, using only enough water to completely dissolve the crystals.
There is, however, a simple way to check the concentration of the lye, without needing to fully dehydrate the potash and then redilute it, and without needing the rather questionable “float an egg” test (where a fresh, pastured chicken egg is put into the lye to see whether it will float partially out of the liquid—at least ¼ of the egg rising above the surface). All that is needed is an accurate kitchen scale and a measuring cup.
STRENGTH OF LYE: THE DENSITY TEST
Density = weight/volume, so find the weight in grams for a given volume of lye liquid. Using metric makes this easier to calculate, so use grams for weight and mL for volume if possible. (Ex. 100 mL of this sample liquid weighs 80 g.)
1. Zero out the kitchen scale with the measuring cup on it, to discount the weight of the measuring cup.
2. Add 100mL of lye to the cup. Record the weight in grams. Divide the weight by 100mL.
The target density for the potash lye is 1.33 g/mL for soap-making (10). If the 100mL sample of lye solution still only weighs 100 grams, keep simmering the lye until it weighs ≥133g per 100mL.
After simmering the lye into a more concentrated solution, it will be more dangerous! When strongly concentrated (1% solution in water), potassium hydroxide has a pH of 13.5 (1), far more caustic than potassium carbonate. BE VERY CAREFUL; this can now burn exposed skin.
To read about soda ash lye, see the next post: Lye Part 3.
Sources:
1) PubChem Database. “Potassium Carbonate.” National Center for Biotechnology Information, 2019. <pubchem.ncbi.nlm.nih.gov/compound/Potassium-carbonate>
2) Butt, John. Daily Life in the Age of Charlemagne. Greenwood Publishing Group, 2002. p.88
3) Rasmussen, Seth. How Glass Changed the World: The History and Chemistry of Glass from Antiquity to the 13th Century. Springer Science & Media, 2012. p.38
4) Babayemi, J.O.; Dauda, K.T.; Nwude, D.O. and Kayode, A. “Evaluation of the Composition and Chemistry of Ash and Potash from Various Plant Materials.” Journal of Applied Sciences, Vol. 10, Issue 16, 2010. p.1820-1824
5) Dunigan et al. “Best Management Practices for Wood Ash as Agricultural Soil Amendment.” Georgia Extension Bulletin 1142, 2002, revised 2016.
6) Etiégni, L; Campbell, A. “Physical and chemical characteristics of wood ash.” Bioresource Technology, 37, 1991. p.173-178
7) Babayemi, J.O.; Adewuyi, G.O.; Dauda, K.T.; and Kayode, A. “The Ancient Alkali Production Technology and the Modern Improvement: A Review.” Asian Journal of Applied Sciences, 4, 2011. p.22-29
8) NIIR Board of Consultants & Engineers. The Complete Technology Book on Wax and Polishes (Reprint). Asia Pacific Business Press Inc, 2011. p. 143
9) Hauschka, Rudolf. The Nature of Substance. Rudolf Steiner Press, 2002. p.143
10) Jake and Katie. “Historical Lye Making, Part 2.” Homestead Laboratory, 2014. <homesteadlaboratory.blogspot.com/2014/02/historical-lye-making-part-2.html>
11) Verberg, Susan. “Of Potash and Lye.” Ithaca, NY, 2015. <academia.edu/27755101/Of_potash_and_lye>
Comments
Post a Comment
Questions and suggestions for further research are welcome. No selling, no trolling, and back up any critique with modern scholarly sources. Comments that do not meet these criteria will be discarded.